Glasgow lab
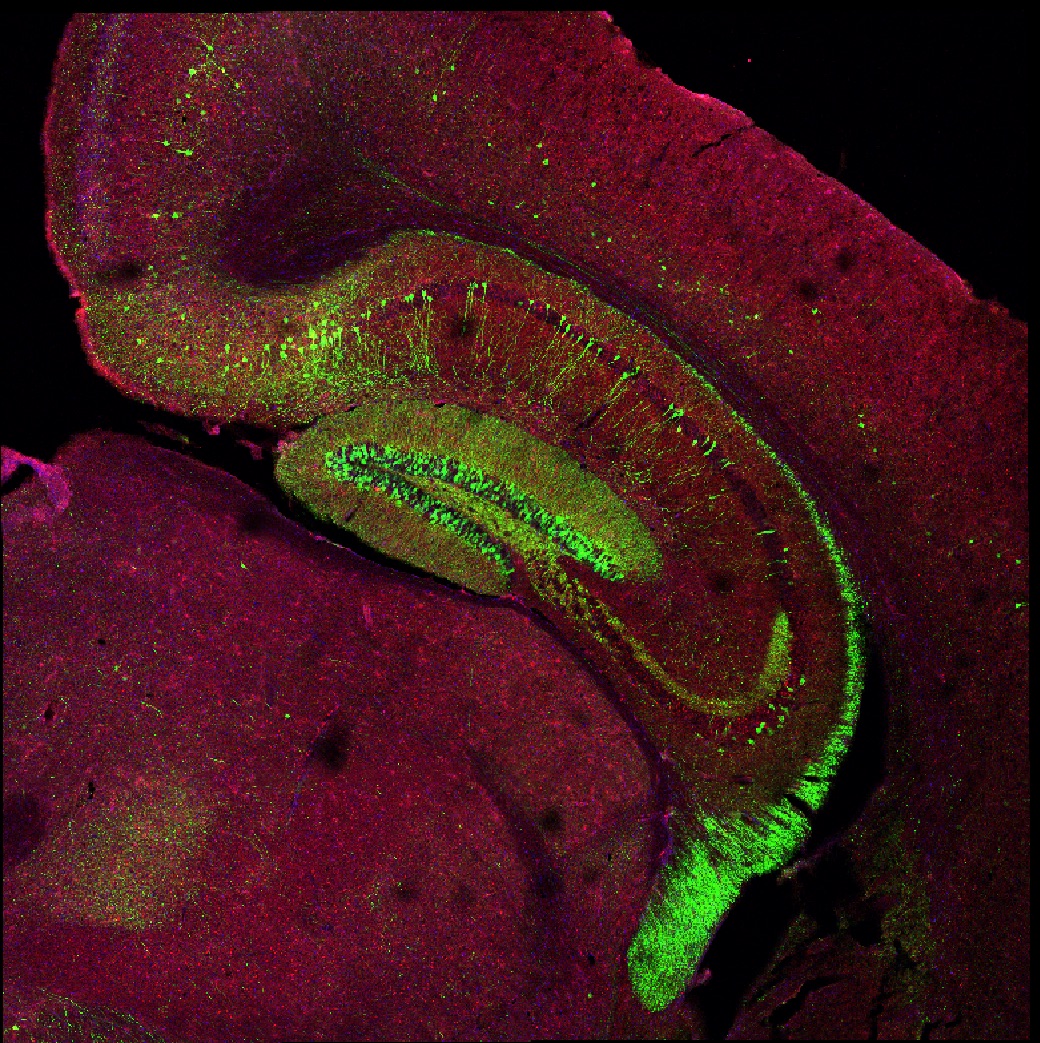
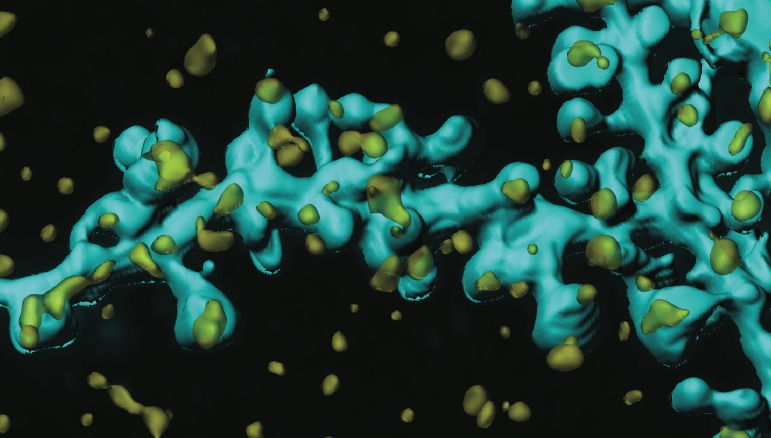
Mission
The primary objective of our research program is to elucidate the biological mechanisms underlying structural and functional synaptic plasticity and, more broadly, to address fundamental unresolved questions of how the mammalian brain consolidates and maintains memories at molecular, cellular, and network levels to adapt and direct on-going movement and behaviour. We employ a combination of scientific techniques, including in vivo and in vitro electrophysiology, optogenetics, live cellular imaging, molecular biology, mouse genetics, and behavioral analyses to understand how the brain consolidates memory.
We are always looking for motivated students and trainees at all levels to join the lab!
Areas of research
Guidance cues
Guidance cues are conserved proteins that are required for neurodevelopment. Interestingly, they are still expressed in the adult brain, and play key roles in synaptic transmission underlying learning and memory formation.
Synaptic plasticity
Learning and memory formation requires the constant reorganization of synapses, highly-specialized sites of chemical and electrical communication in the brain. This involves not only molecular shuffling but also structural changes at the level of individual neurons.
Neuromodulation
How glutamatergic excitatory synapses function can be heavily modulated by elements outside the synapse proper – namely by changes in neuromodulatory tone. Changes in intrinsic excitability driven by these neuromodulators may alter how circuits emerge and function.
Spatial memory
Changes in how brain cells communicate with one another can impact network function that underlies memory formation. These synaptic alterations can result in different spatial representations, called cognitive maps, but how guidance cues impact these maps remains unclear.
Publications
Google Scholar : https://scholar.google.ca/citations?user=YRlJn1sAAAAJ
Reprints available – please email sglasgow (at) brocku.ca
- Feighan, K. M., Thakare, H. K., Glasgow, S. D., & Kennedy, T. E. Convergence and divergence of molecular mechanisms in Hebbian and homeostatic plasticity. Frontiers in Synaptic Neuroscience. 18.
- Glasgow, S. D., Wong, E. W., Beamish, I. V., Lancon, K., Gibon, J., Séguéla, P., Ruthazer, E. S., & Kennedy, T. E. (2024) Acetylcholine synergizes with netrin-1 to drive persistent firing in the entorhinal cortex. Cell Reports. 43 (2). https://www.cell.com/cell-reports/pdf/S2211-1247(24)00140-2.pdf
- Clement, J. P., Al-Alwan, L., Glasgow, S. D., Stolow, A., Ding, Y., Quevedo Melo, T., Khayachi, A., Liu, Y., Hellmund, M., Haag, R., Milnerwood, A., Grutter, P., & Kennedy, T. E. (2022) Dendritic polyglycerol amine: An enhanced substrate to support long-term neural cell culture. ASN Neuro. 14 (1) 1-17. https://journals.sagepub.com/doi/abs/10.1177/17590914211073276
- Glasgow, S. D., Ruthazer, E. S., & Kennedy, T. E. (2021) Guiding synaptic plasticity: novel roles for netrin-1 in synaptic plasticity and memory formation in the adult brain. Journal of Physiology. 599 (2). https://physoc.onlinelibrary.wiley.com/doi/abs/10.1113/JP278704
- Glasgow, S. D., Wong, E. W., Thompson-Steckel, G., Séguéla, P., Ruthazer, E. S., & Kennedy, T. E. (2020) Pre- and post-synaptic roles for DCC in memory consolidation in the adult mouse hippocampus. Molecular Brain. 13 (56) 1-20. https://link.springer.com/article/10.1186/s13041-020-00597-2
- Glasgow, S. D., McPhedrain, R., Madranges, J. F., Kennedy, T. E., & Ruthazer, E. S. (2019) Approaches and limitations in the investigation of synaptic transmission and plasticity. Invited, Frontiers in Synaptic Neuroscience. 11 (20) 1-16. https://www.frontiersin.org/articles/10.3389/fnsyn.2019.00020/
- Wong, E. W.*, Glasgow, S. D.*, Trigiani, L. J., Chitsaz, D., Rymar, V., Sadikot, A., Ruthazer, E. S., Hamel, E., & Kennedy, T. E. (2019) Spatial memory formation requires netrin-1 expression by neurons in the adult mammalian brain. Learning and Memory. 26 (3), 77-83. * indicates equal contribution / co-first author. http://learnmem.cshlp.org/content/26/3/77
- Glasgow, S. D., Labrecque, S., Beamish, I. V., Aufmkolk, S., Gibon, J., Han, D., Harris, S. N., Wiseman, P. W., McKinney, R. A., Séguéla, P., De Koninck, P., Ruthazer, E. S., & Kennedy, T. E. (2018) Activity-dependent netrin-1 secretion drives synaptic insertion of GluA1-containing AMPA receptors in the hippocampus. Cell Reports. 25 (1), 168-182.e6 https://www.cell.com/cell-reports/pdf/S2211-1247(18)31458-X.pdf
- Boyce, R., Glasgow, S. D., Williams, S., & Adamantidis, A. R. (2016) Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 352 (6287), 812-816. https://www.science.org/doi/abs/10.1126/science.aad5252
- Munz, M., Gobert, D., Higenell, V., Van Horn, M. R., Glasgow S. D., Schohl, A., & Ruthazer, E. S. (2014) Using two-photon intravital imaging to study developmental plasticity of neural circuits. Microscopy and Microanalysis. 20 (S3), 1342-1343.
- Goldman, J., Ashour, M., Magdesian, M., Tritsch, N., Christofi, N., Chemali, R., Stern, Y., Thompson-Steckel, G., Harris, S., Gris, P., Glasgow, S. D., Grutter, P., Bouchard, J-F., Ruthazer, E. S., Stellwagen, D., & Kennedy, T. E. (2013) Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. Journal of Neuroscience. 33 (44), 17278-89. https://www.jneurosci.org/content/33/44/17278.short
- Jego, S., Glasgow, S. D., Gutierrez-Herrara, C., Ekstrand, M., Reed, S. J., Boyce, R., Friedman, J., Burdakov, D., & Adamantidis, A. R. (2013). Optogenetic identification of a rapid-eye movement (REM) sleep modulatory circuit in the hypothalamus. Nature Neuroscience. 16 (11): 1637-43. https://www.nature.com/articles/nn.3522
- Horn, K. E., Glasgow, S. D., Gobert, D., Bull, S. J., Luk, T., Girgis, J., Tremblay, M. E., McEachern, D., Bouchard, J. F., Haber, M., Hamel, E., Krimpenfort, P., Murai, K. K., Berns, A., Doucet, G., Chapman, C. A., Ruthazer, E. S., Kennedy, T. E. (2013). DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Reports. 3: 173-185. https://www.cell.com/cell-reports/pdf/S2211-1247(12)00429-9.pdf
- Glasgow, S. D., & Chapman, C. A. (2013). Muscarinic depolarization of layer II neurons of the parasubiculum.. PLoS One. 8 (3): 1-14. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0058901
- Glasgow, S. D., Glovaci, I., Karpowicz, L., & Chapman, C. A. (2012). Cholinergic suppression of excitatory synaptic transmission in layers II/III of the parasubiculum. Neuroscience. 201 (1) 1-11. https://www.sciencedirect.com/science/article/pii/S0306452211013248
- Glasgow, S. D., & Chapman, C. A. (2008). Conductances mediating intrinsic theta-frequency membrane potential oscillations in layer II parasubicular neurons. Journal of Neurophysiology. 100 (5): 2746-56. https://journals.physiology.org/doi/abs/10.1152/jn.90351.2008
- Kourrich, S., Glasgow, S. D., Caruana, D. A., & Chapman, C. A. (2008). Postsynaptic signals mediating induction of long-term synaptic depression in the entorhinal cortex. Neural Plasticity. 2008:840374. https://www.hindawi.com/journals/NP/2008/840374/
- Glasgow, S. D., & Chapman, C. A. (2007). Local generation of theta-frequency EEG activity in the parasubiculum. Journal of Neurophysiology. 97 (6): 3868-3879. https://journals.physiology.org/doi/abs/10.1152/jn.01306.2006
- Bland, B.H. DeClerck, S., Jackson, J., Glasgow, S. D., and Oddie, S.D. (2007) Septohippocampal properties of N-Methyl-D-Aspartate–induced theta-band oscillation and synchrony. Synapse. 61 (3): 185-197.
Book chapters
- Glasgow, S. D., Gutierrez Herrera, C., & Adamantidis, A. R. (2017) Behavioral phenotyping using optogenetic technology. In Neuro-Phenome: Cutting-edge Approaches and Technologies in Neurobehavioral Genetics (V. Tucci, Ed.). Oxford: Wiley-Blackwell. Invited.
Team members
Stephen D. Glasgow, PhD


Emily Kacur, PhD candidate

Harshit Thakare, MSc student

Gabryelle Corriveau, MSc student (co-supervised by R. Macpherson)

- Current undergraduate students (2025-26)
- Ethan Busch, BSc Honours (Neuroscience)
- Nick Jessome, volunteer (Biological Sciences)
Lab alumni
- Fiona O’Farrell, BSc Honours (Neuroscience, 2024-25), NSERC USRA student, current: Pharmacy assistant
- Emma Regudo, BSc Honours (Neuroscience, 2024-25), current: Brain injury rehabilitation coordinator (Niagara)
- Gabryelle Corriveau, BSc Honours (Neuroscience, 2024-25), current: MSc student in the lab
- Zoe Gagnon, MSc (Biological Sciences, 2023-2024), current: MSEd student at Niagara University
- Madeleine Pollett, BSc Honours (Neuroscience, 2023-24), current: MSc student in McPherson lab
- Harshit Thakare, BSc Honours (Neuroscience, 2023-24), current: MSc student in the lab
- John Hansler, BSc Honours (Neuroscience, 2022-23), current: academic tutor
- Emily Kacur, BSc Honours (Neuroscience, 2022-23), current: PhD candidate in the lab
- Izma Bokhari, FMS Mentorship student, current: medical student in St. Kitts
